Bridging the Gap: Assessing Hypertension Drug Availability and Alignment with Malaysian CPG Guidelines and MOH Formulary

This study aims to provide a comprehensive comparison of hypertension drugs registered in Malaysia through the Malaysia Drug Registration (NPRA Product Registration) [1], the official hypertension management as outlined in the CPG [2], and the MOH's official drug formulary. By examining the consistency and alignment among these three sources, we seek to evaluate the accessibility and availability of essential hypertension medications in Malaysia.
This analysis will not only shed light on the current state of hypertension treatment in the country but also identify potential gaps and areas for improvement to ensure that patients receive the most effective and affordable care possible. The objective of the study is as below:
a) Identifying gaps and inconsistencies: Comparing the product registered can help identify gaps or inconsistencies between these sources. By uncovering these disparities, stakeholders can take necessary steps to address these issues and ensure better alignment across the different sources, ultimately improving hypertension management in Malaysia.
b) Improved patient care: Ensuring that the drugs recommended by the CPG are available and accessible to patients is essential for optimal patient care. By analyzing the availability of these drugs in the NPRA product registration and the MOH drug formulary, healthcare providers and policymakers can make more informed decisions about the appropriate medications for hypertension management.
c) Cost-effective treatment: Analyzing the availability of generic drugs in the registered drug and MOH drug formulary can help evaluate the affordability of hypertension treatment options. Identifying and promoting cost-effective treatment options can help reduce the financial burden on patients and the healthcare system.
Methodology
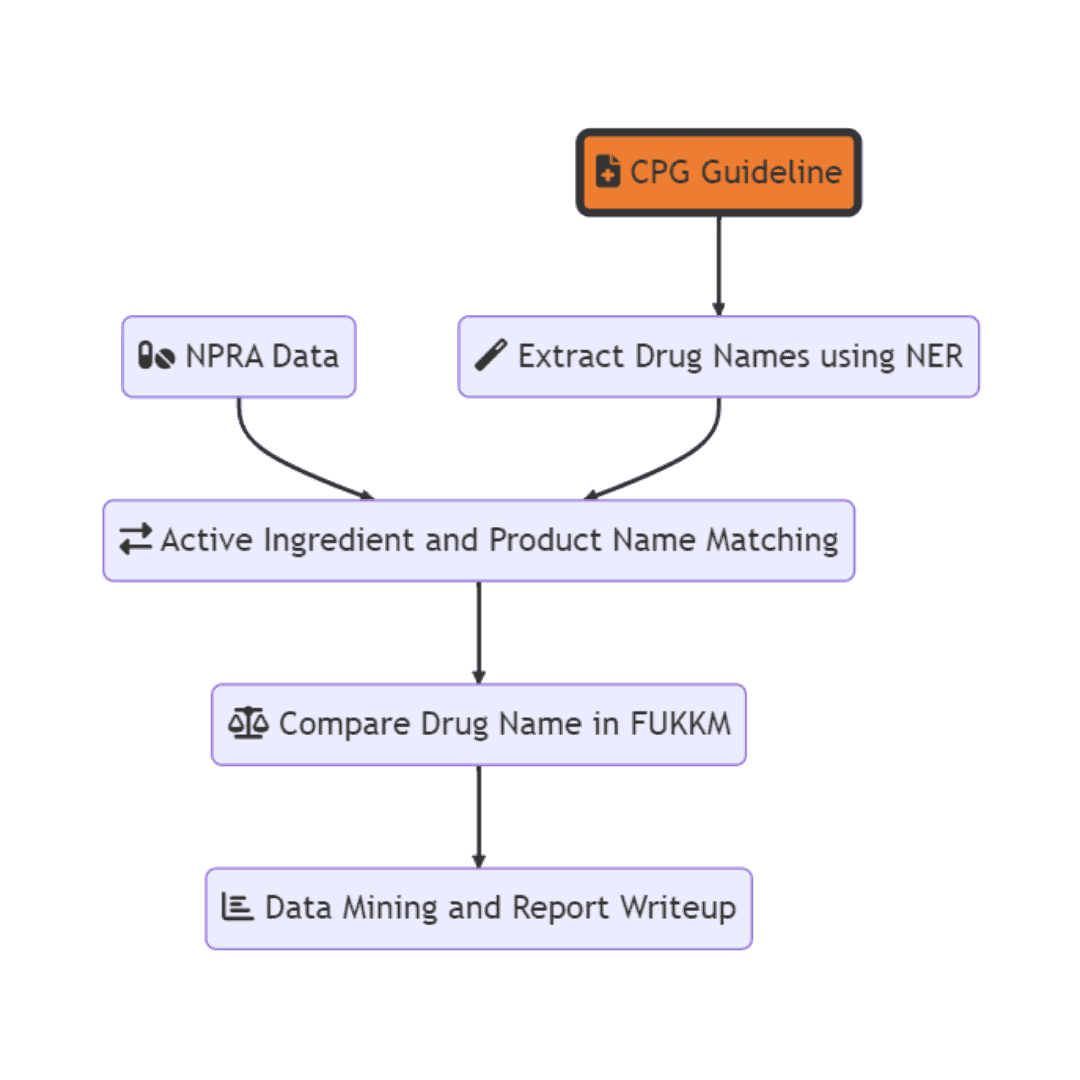
The first step involves using NER (named entity recognition) knowledge extraction techniques to identify drug names from the clinical practice guidelines. This step is important because it allows for the automatic identification of drugs mentioned in the guidelines, which can then be used for comparison with the registered drugs in Malaysia. The drug name is then classified into drug class and drug name for comparison.

In this step, each drug name or drug class is identified and each mentioned in the CPG guideline is counted. The idea is the more frequently the drug is mentioned in CPG, the more emphasize it is in the CPG.
The second step involves gathering data from the NPRA (National Pharmaceutical Regulatory Agency) on the drugs that are registered in Malaysia. This dataset will serve as the basis for comparison with the drug names identified from the clinical practice guidelines. This step allows for the identification of any potential matches or discrepancies between the drugs recommended in the guidelines and those that are actually registered in Malaysia.
The next step involves comparing the mentioned drug name to the MOH’s Formulary and the availability is marked in the dataset.
Finally, data mining and report writeup are performed to summarize the findings of the analysis. This includes identifying which drugs were most frequently mentioned in the guidelines, which drugs had the biggest discrepancies between the guidelines and registered drugs, and any potential implications of these findings for patient care and healthcare equity.
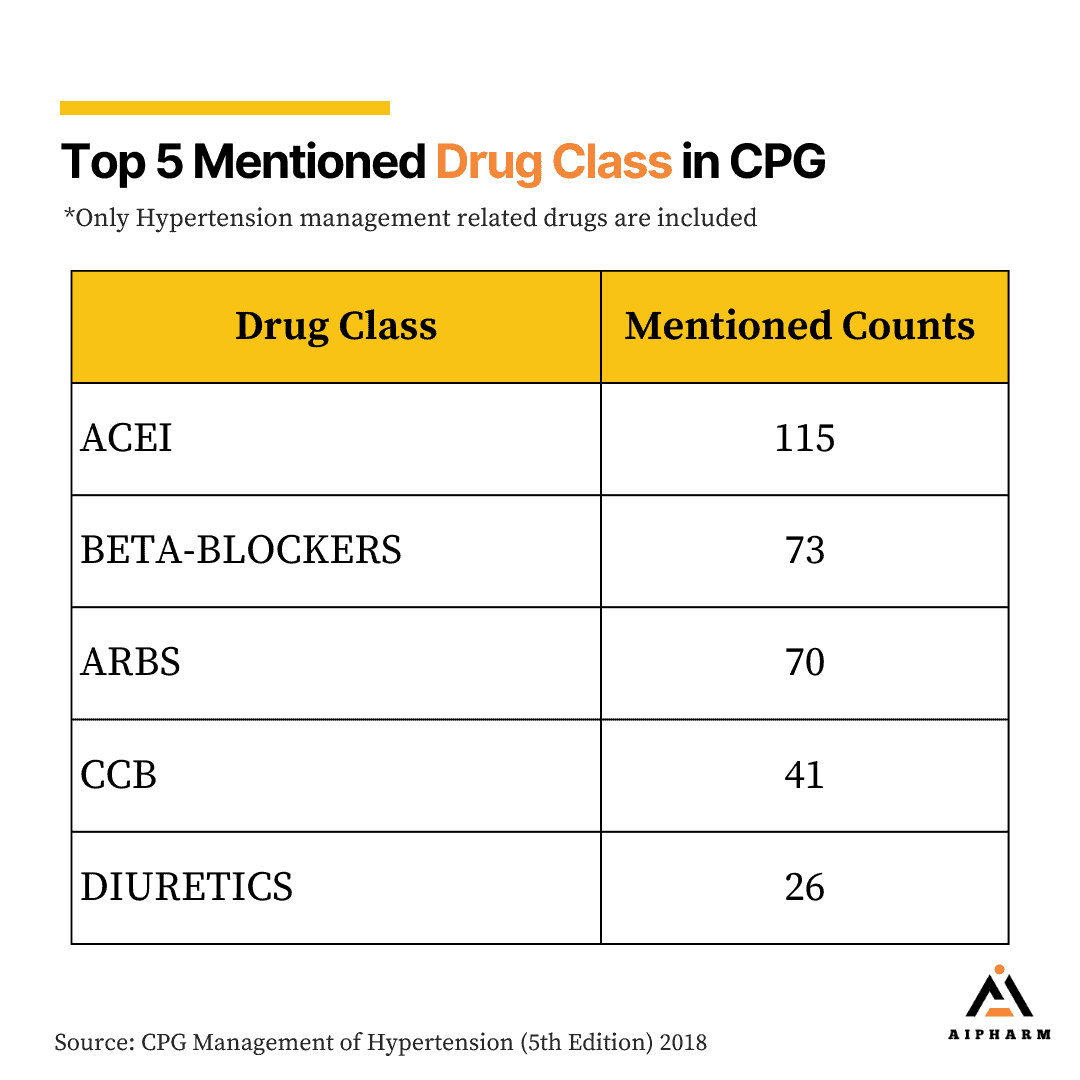
Top 5 mentioned Drug Classes in Hypertension Management CPG
Top 5 mentioned Drug Names in Hypertension Management CPG
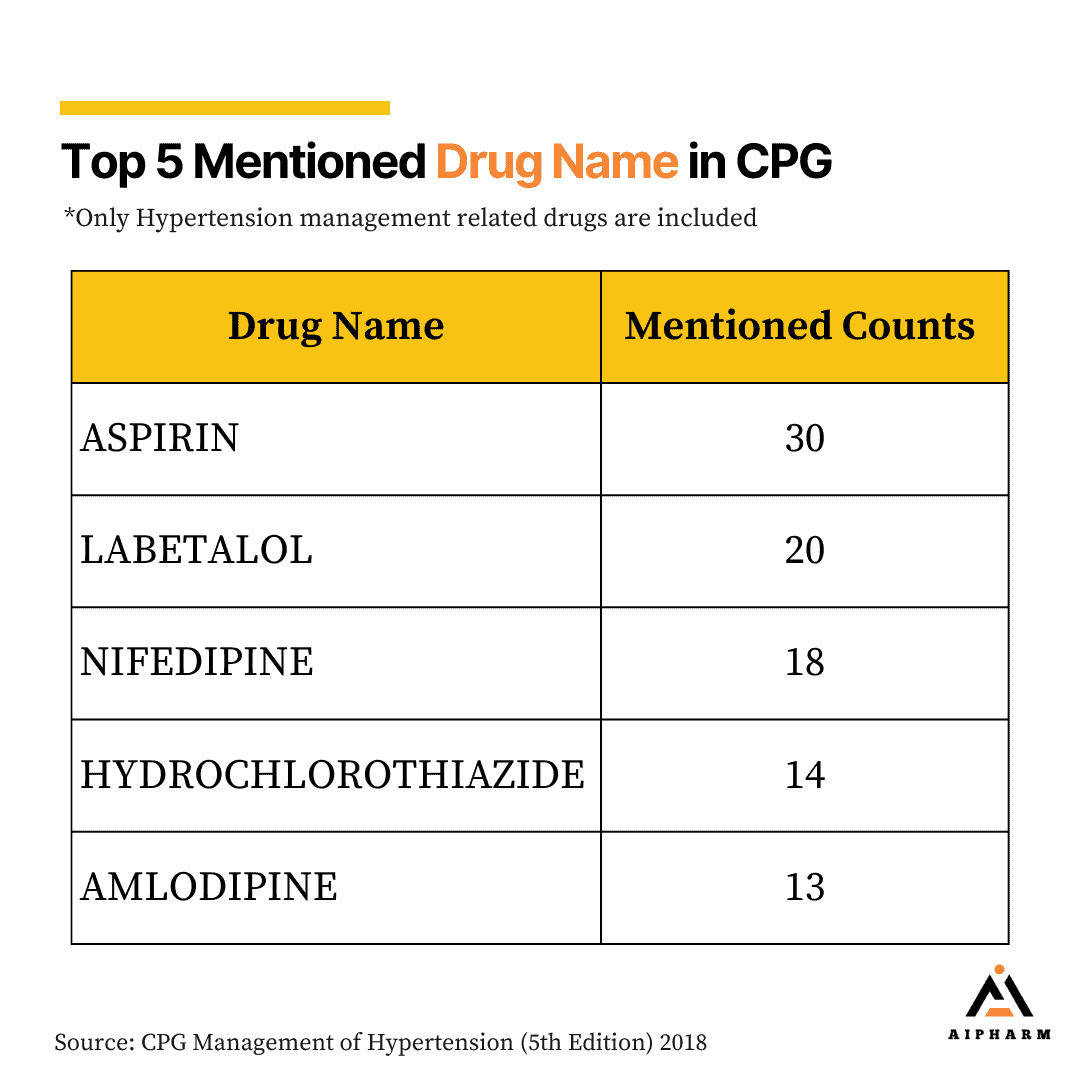
One interesting finding is that the most mentioned drug in the management of hypertension is Aspirin (an anti-platelet), it is heavily mentioned due to its role in the prevention of cardiovascular events, particularly in high-risk patients with hypertension. There is a special section in the CPG which specially mention the use of aspirin in hypertension management. The rest of the drug falls into the category of most mentioned drug classes.
CPG vs Generic Drugs Availability
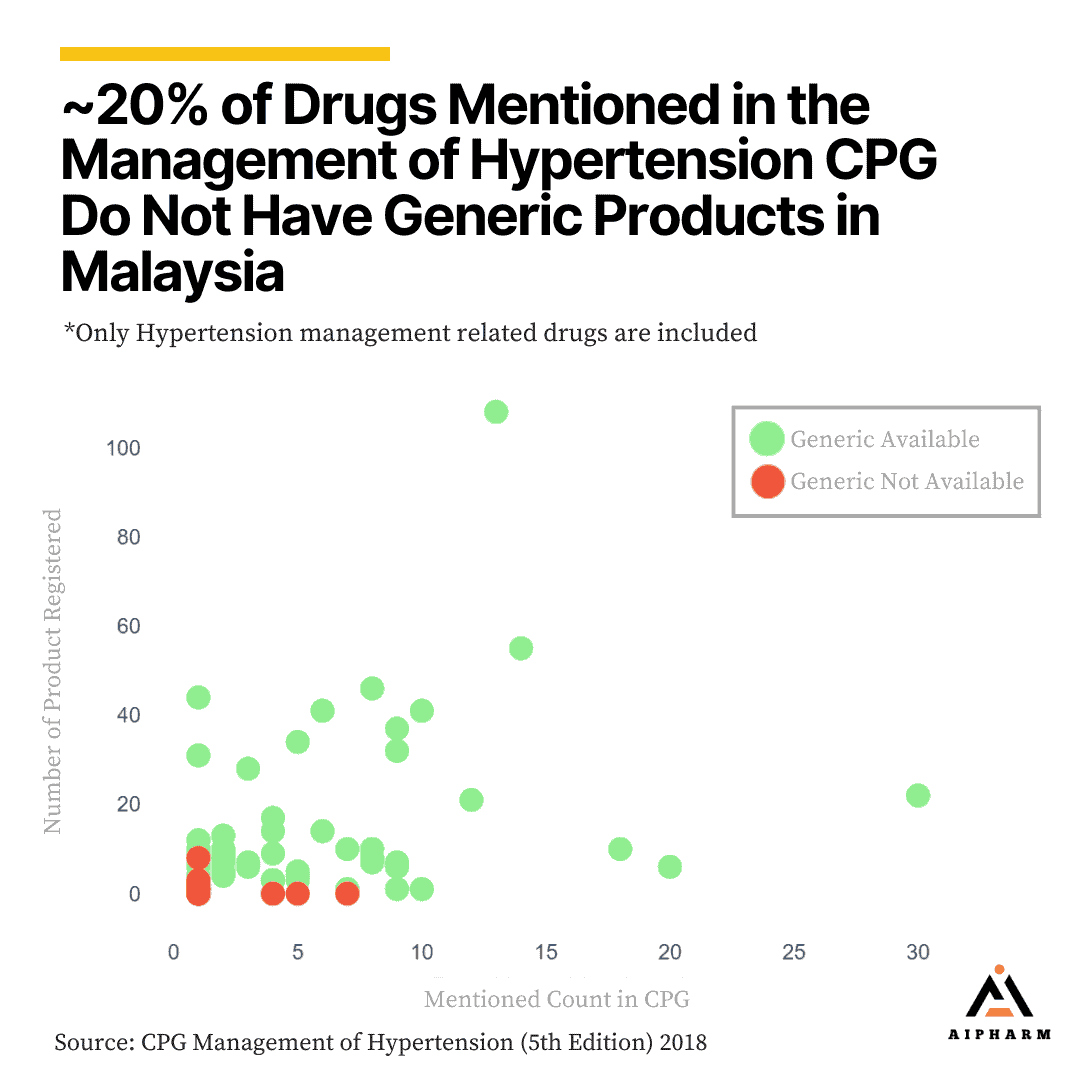
We further compare the data to generic availability based on the registered drug, surprisingly out of 69 drugs mentioned, around 20% of the drugs lack generic alternatives in the country. The absence of generic versions for a significant portion of recommended drugs can limit the affordability and accessibility of essential hypertension medications for patients, especially those from lower-income backgrounds.
Generic drugs are typically less expensive than their brand-name counterparts, making them a more cost-effective option for managing chronic conditions like hypertension.
The lack of generic alternatives may also strain the healthcare system, as higher drug costs can lead to increased overall healthcare expenditures. This issue highlights the need for policymakers and stakeholders to address the gap in generic drug availability by encouraging the development and registration of generic versions of the recommended drugs.
CPG vs MOH Formulary
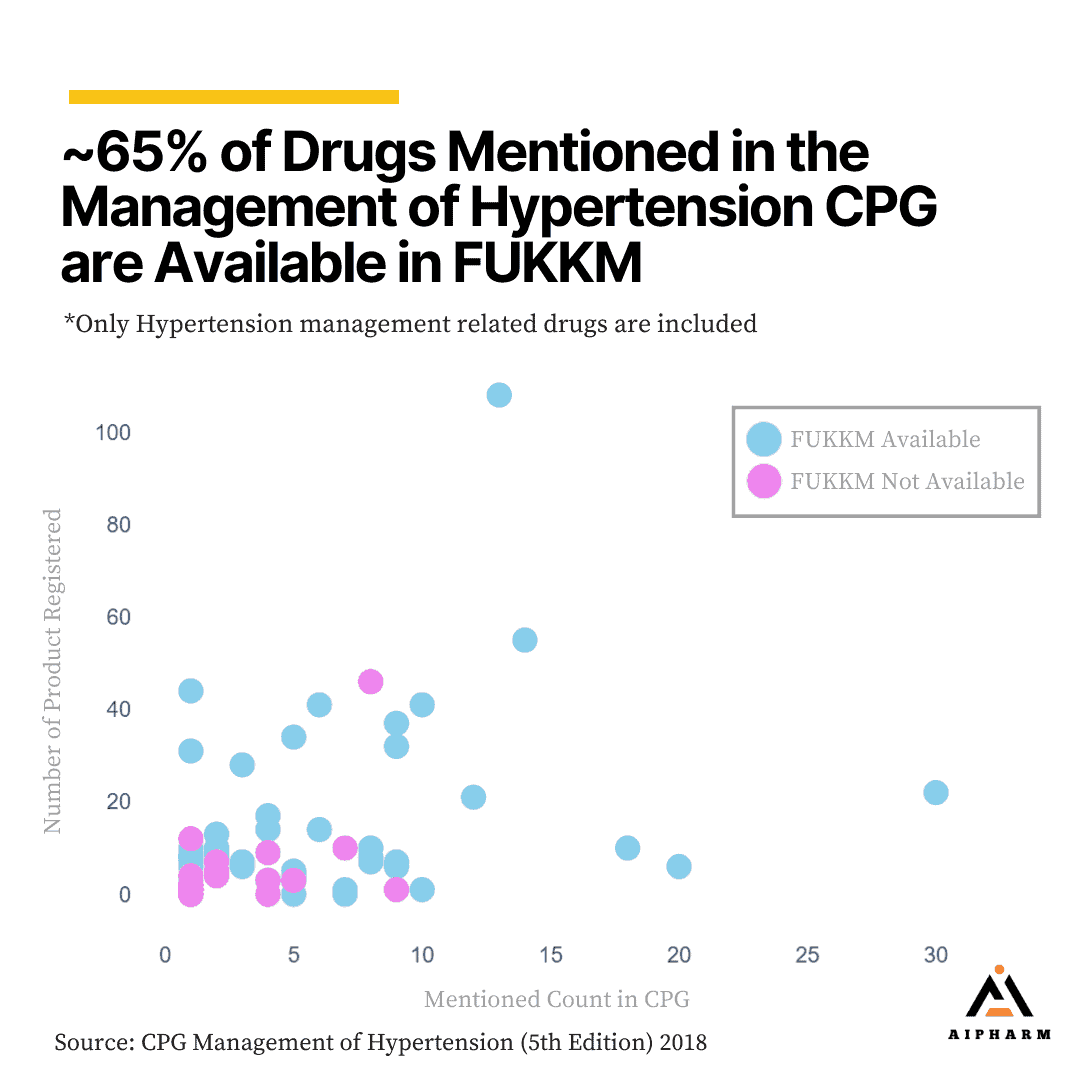
Our findings also show that around 65% of the drugs mentioned in the management of hypertension CPG are available in the national drug formulary. It shows positive coverage where a majority of the recommended drugs are included in the national drug formulary. This ensures that many of the evidence-based treatment options for hypertension are accessible.
However, the remaining 35% of the drugs mentioned in the CPG not being available in the national drug formulary indicates that there is still room for improvement. The unavailability of these drugs may limit treatment options for healthcare providers and patients, potentially impacting the management of hypertension.
Since there are disparities between drugs mentioned in CPG and the drug formulary, continuous monitoring and evaluation of the alignment between CPG recommendations and the national drug formulary should be enabled. This can help track progress and measure the impact of any policy changes or interventions aimed at improving the availability of recommended drugs. Lastly, greater transparency in listing decisions could help address these discrepancies and improve alignment between these sources. [4]
Overall, while the availability of 65% of the CPG-recommended drugs in the national drug formulary is a positive sign, the findings suggest that there is still room for improvement to ensure better alignment and access to the most effective hypertension treatments for all patients.
Let’s zoom in on the drugs not registered in Malaysia:
Hypertension Drugs in CPG that are not registered in Malaysia
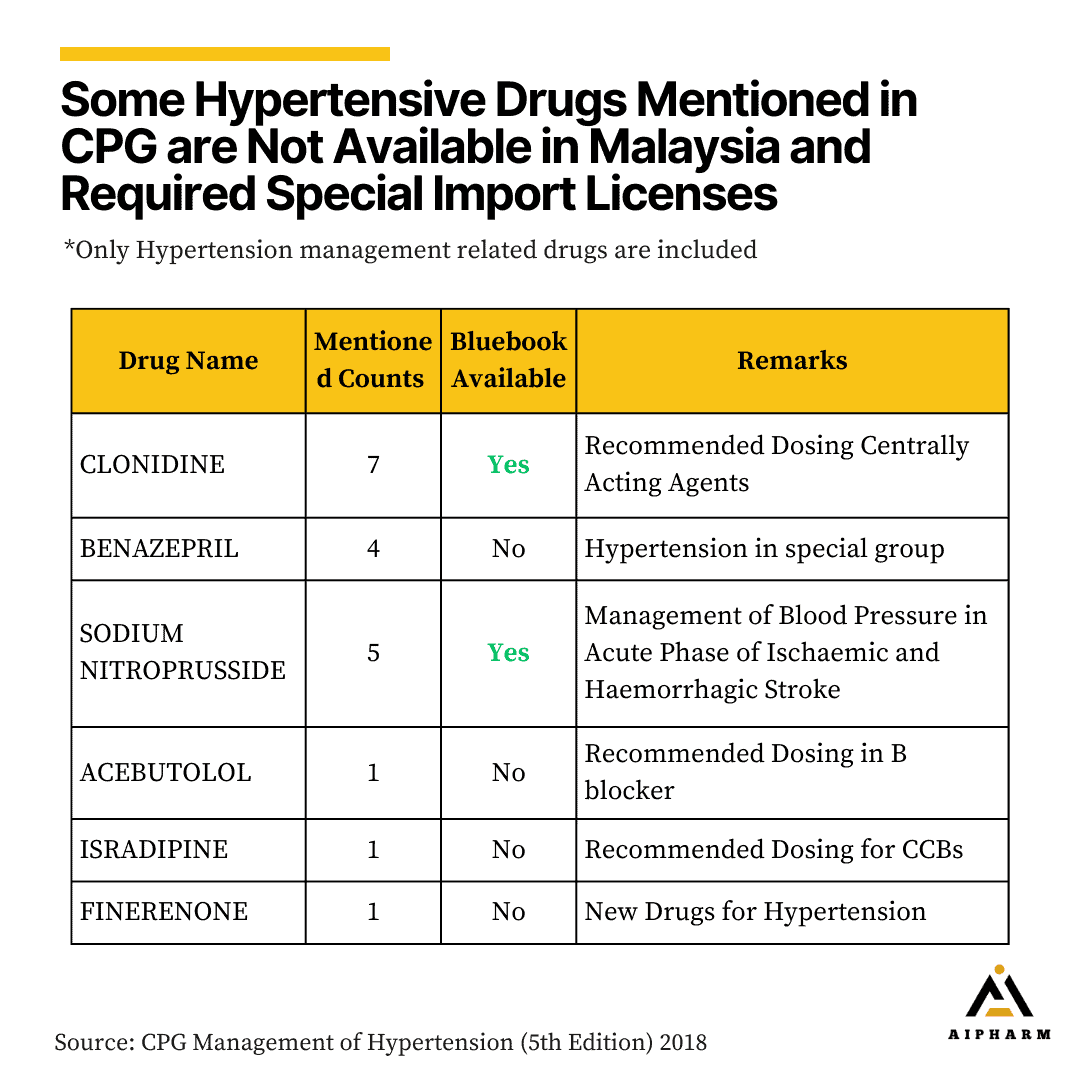
Overall, there are only 6 drugs that are mentioned doesn’t register in Malaysia. To our surprise, Clonidine and Sodium Nitroprusside are not registered in Malaysia but are available as Formulary items.
Out of our curiosity, we go to survey how these medicines can be used either in private or government settings:
1. Use of Unregistered Drugs in Government Settings [3]
For government health facility setting, it falls to the procedure of applying for approval from the Director-General of Health (KPK) or Senior Director of Pharmaceutical Services Division (PKPF) is required under r.15(6) of Control of Drugs and Cosmetics Regulations 1984
2. Use of Unregistered Drugs in Private Settings [5]
In order for a private hospital to import unregistered drugs the drug must fall into life-saving medicine and the facility has to apply for an import permit via the Pharmaceutical Service Division (PSD) via Form BPF/213-1
According to our inquiry from a private hospital pharmacist, the cost of the medicine that is imported is more expensive and could be more than 2-fold in price after factoring in the shipping cost and currency exchange. Additionally, the import permit took time to be approved and shipping time is counted. These factors greatly increase the burden on the patients.
We do understand that it falls into business consideration whenever it comes to drug availability and drug registration. But upon further reasoning and investigation, we found out that if a local manufacturer or a local importer wants to manufacture or register the drugs in Malaysia, they don’t have easy access to data on the usage, such as, for this case, how many Sodium Nitroprusside had been used in the nation yearly or what are the most frequent applied KPK items. The only data that can be found is in 2022 when NPRA published the usage and application count for the permit application in the year 2021.
Having access to these data can guide better decision-making for local healthcare entrepreneurs to decide whether to import or manufacture the generic product in Malaysia and with more transparent data, we can provide a better quality of healthcare to Malaysian.
Lastly, some drug recommended in CPG exert their unique therapeutic effects, for example, benazepril [6] shows to improve compliance in the elderly and the combination of benazepril with amlodipine leads to a reduction of cardiovascular events in the overall study population as well as the diabetic subgroup
Words from Authors
In conclusion, this study provides a comprehensive analysis of hypertension drug availability and alignment with Malaysian CPG guidelines and the MOH formulary. Our findings reveal that while there is a significant overlap between the CPG-recommended drugs and those available in the national drug formulary, there is still room for improvement to ensure better alignment and access to the most effective hypertension treatments for all patients.
By addressing the identified gaps and inconsistencies between the CPG guidelines, NPRA product registration, and MOH formulary, stakeholders can work together to optimize hypertension management in Malaysia. This, in turn, will contribute to better patient care, cost-effective treatment options, and a more equitable healthcare system for all Malaysians.
If you are interested in the data we compiled, it is available here.
References
[1] Quest 3 by NPRA
[2] MOH Management of Hypertension CPG
[4] Explain Why Drugs Rejected From Formulary, US Pharma Tell Malaysia
